Abstract
Background: Acute myeloid leukemia (AML) is an aggressive blood cancer with a wide range of response and relapse rates using standard chemotherapy combining anthracycline plus cytarabine (7+3). The stem cell receptor tyrosine kinase KIT is expressed on more than 10% of blasts in 95% of relapsed AML cases and mediates leukemic proliferation and has anti-apoptotic effects (Domen and Weissman 2000). AML with high KIT expression is associated with poorer outcome (Del Poeta, Venditti et al 2003).
Goals: To study the efficacy and safety of combination 7+3 and nilotinib in patients (pts) with AML and KIT expression. Primary goal is to determine the complete response (CR) rate; while secondary goals include 2-year overall survival (OS) and disease free survival (DFS) in addition to safety.
Methods: A single arm, Phase II study, enrolled pts at Mayo Clinic (MN and AZ). Appropriate IRB was obtained and study was registered (NCT 01806571). Pts were enrolled if they were newly diagnosed with AML with KIT (CD117) expression of ≥ 20% on myeloblasts by flow cytometry. KIT mutations were allowed. Nilotinib 300 mg twice daily was given on days 4-14 of induction and consolidation; and continuous daily maintenance therapy for up to 2 years. Cytarabine 100 mg/m2/day continuous IV x7 days plus daunorubicin 60 mg/m2 IV daily x3 days were used for induction, while consolidation used standard cytarabine 3 gm/m2 twice daily days 1, 3, 5 for a total of 4 cycles. This is a Simon 1-stage design with a safety analysis after enrolling 12 pts, and an interim analysis after enrolling 18 out of 43 pts (Al-Kali, ASH 2015) recommended to continue study accrual.
Results:
i)- Demographics:
Thirty four pts were enrolled from July 2013 to June 2017. Median age was 59 years (range 24-69) with 71% being male. Median laboratory findings include hemoglobin of 8.8 gm/dL, platelets of 56 x109/L, white blood count of 3.3 x109/L (0.4-125), and peripheral blood blasts 17 %(0-94%). Cytogenetics were normal in 43% of the pts and favorable cytogenetics were seen in 6%(inv 16). FLT3 gene testing was done on 26 pts and was positive in 13%. KIT gene sequencing (exon 8, 9, 10, 11, 17) revealed pathogenic mutation in 1/28 cases (4%).
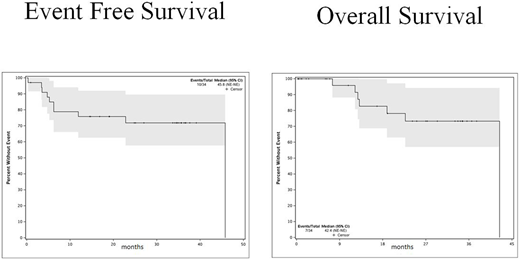
ii)- Clinical outcome
Out of all 34 pts enrolled on the study, 18 (53%) achieved CR (or CR with incomplete platelet recovery) with a median CR duration of 21.8 months. Of 26 evaluable pts, the overall CR rate was 69%. 4 of the 18 pts (22%) who achieved remission needed a second induction. One pt died due to liver failure (had only one dose of nilotinib and toxicity was attributed to daunorubicin). 13 (38%) pts proceeded to allogeneic stem cell transplant (HSCT), 12 of whom are alive and none were able to initiate nilotinib maintenance. Only 6 (1 had HSCT) out of 34 (18%) pts relapsed after achieving CR. Median DFS was 45.8 months, while median OS was 42.4 months. 2-year DFS and OS were 58% and 72%, respectively.
iii)- Safety
Thirty four pts were evaluated for adverse events (AE). Fourteen pts had G4 non-hematological AEs, including fourteen G4 AEs related to infection, 2 with electrolyte imbalances, 1 heart failure, 1 elevated bilirubin, 1 elevated lipase, and 1 jejunal hemorrhage. One patient had G5 liver failure. Most common (>20%) G3 non-hematological AEs were febrile neutropenia (56%), hypophosphatemia (21%), elevated ALT (21%) and hypertension (21%).
Conclusion: Combination daunorubicin and cytarabine with nilotinib (DATA) appears to be safe and effective. Final results show an acceptable safety profile with most common AE being infection. Thirty day mortality was low (3%). DATA regimen has comparable CR rates of 53% (intent to treat) and 69% in evaluable pts. Relapse rates were very low at 18% with durable responses and encouraging survival rates.
Al-Kali:Novartis: Research Funding. Tibes:Novartis: Research Funding. Palmer:Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal